Straight lines that appear crooked
Straight lines that appear crooked
Straight lines that appear crooked
Straight lines that appear crooked
Genetics
Family history3 (genetic predisposition)
Genetics
Family history3 (genetic predisposition)
Genetics
Family history3 (genetic predisposition)
Genetics
Family history3 (genetic predisposition)
There is an FDA-approved treatment option for GA secondary to AMD
There is an FDA-approved treatment option for GA secondary to AMD
There is an FDA-approved treatment option for GA secondary to AMD
Out of 201 eyes, 75 eyes were coded as early or intermediate AMD5*
But when using the Hoover categorization
did not have GA
47%
(n=35/75)

had GA
53%
(n=40/75)
See how GA may impact your patients
Learn more about an FDA-approved treatment option for GA secondary to AMD
GA and AMD
GA is the advanced form of dry age-related macular degeneration (AMD), and progression to GA is a frequent outcome of dry AMD.4,7-10
Click on the tabs below to learn more.
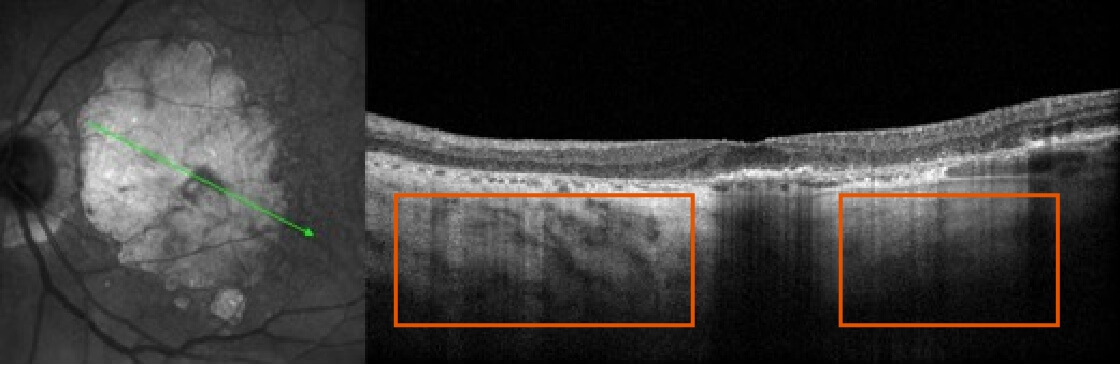
Optical Coherence Tomography (OCT) B-scan
Healthy OCT B-scan
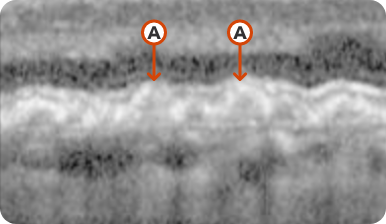

Few medium-sized drusen
Healthy OCT B-scan


Few medium-sized drusen

Hyperreflective foci representing RPE migration
Healthy OCT B-scan


Few medium-sized drusen
Healthy OCT B-scan


Few medium-sized drusen

Hyperreflective foci representing RPE migration
Images may vary based on different device manufacturers and imaging platforms.
What to look for:
- Drusenoid PED, hyper-reflective foci, and reticular pseudodrusen are indicative of areas at high risk for developing atrophy7
- Loss of RPE as well as photoreceptor and choriocapillaris layers7
- Increase in choroidal hypertransmission indicative of atrophy7
- Drusen collapse is a precursor to atrophy9
Considerations:
- Cross-section needed for accurate recognition10
- Can provide early GA diagnosis11
- Scans are in high resolution11
- Challenges in image interpretation can lead to longer reading times12
Optical Coherence Tomography (OCT) en face and Near Infrared Reflectance (NIR)
If you do not have access to FAF imaging, OCT en face can be used as a substitute to visualize the full extent of GA lesions. On certain machines, NIR images can be acquired simultaneously with OCT en face images. Using both OCT en face and NIR scans allows for the highest accuracy in detecting RPE indicative of GA.7,13
Healthy OCT en face Image*

OCT en face Image 1


Multifocal lesion appears as a bright area due to increased penetration of light into the choroid caused by RPE and outer retina atrophy14
OCT en face Image 2


Progression of larger multifocal lesion with subfoveal involvement14
Healthy NIR Image

NIR Image 1*


Multifocal lesion appears as a bright area due to increased penetration of light into the choroid caused by RPE and outer retina atrophy14
NIR Image 2*


Multifocal lesion appears as a bright area due to increased penetration of light into the choroid caused by RPE and outer retina atrophy14
Images may vary based on different device manufacturers and imaging platforms.
Images may vary based on different device manufacturers and imaging platforms.
What to look for:
- Distinct borders, location, and size of lesion7,13
- Lesions appear hyperreflective on NIR and OCT en face images vs hyporeflective on FAF images7,13
Considerations:
- Allows for more comprehensive visualization of GA lesion borders in the absence of FAF7,10,12
- OCT en face images can guide you in selecting the proper cross-section for OCT B-scans6,7
- Interpretation dependent on imaging quality7
Optical Coherence Tomography (OCT) B-scan
Healthy OCT B-scan


Few medium-sized drusen
Healthy OCT B-scan


Few medium-sized drusen

Hyperreflective foci representing RPE migration
Healthy OCT B-scan


Few medium-sized drusen
Healthy OCT B-scan


Few medium-sized drusen

Hyperreflective foci representing RPE migration
Images may vary based on different device manufacturers and imaging platforms.
What to look for:
- Drusenoid PED, hyper-reflective foci, and reticular pseudodrusen are indicative of areas at high risk for developing atrophy7
- Loss of RPE as well as photoreceptor and choriocapillaris layers7
- Increase in choroidal hypertransmission indicative of atrophy7
- Drusen collapse is a precursor to atrophy9
Considerations:
- Cross-section needed for accurate recognition10
- Can provide early GA diagnosis11
- Scans are in high resolution11
- Challenges in image interpretation can lead to longer reading times12
Optical Coherence Tomography (OCT) B-scan
Healthy OCT B-scan


Few medium-sized drusen
Healthy OCT B-scan


Few medium-sized drusen

Hyperreflective foci representing RPE migration
Healthy OCT B-scan


Few medium-sized drusen
Healthy OCT B-scan


Few medium-sized drusen

Hyperreflective foci representing RPE migration
Images may vary based on different device manufacturers and imaging platforms.
What to look for:
- Drusenoid PED, hyper-reflective foci, and reticular pseudodrusen are indicative of areas at high risk for developing atrophy7
- Loss of RPE as well as photoreceptor and choriocapillaris layers7
- Increase in choroidal hypertransmission indicative of atrophy7
- Drusen collapse is a precursor to atrophy9
Considerations:
- Cross-section needed for accurate recognition10
- Can provide early GA diagnosis11
- Scans are in high resolution11
- Challenges in image interpretation can lead to longer reading times12
-
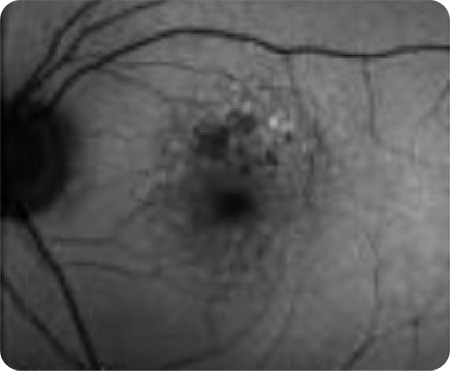
Small 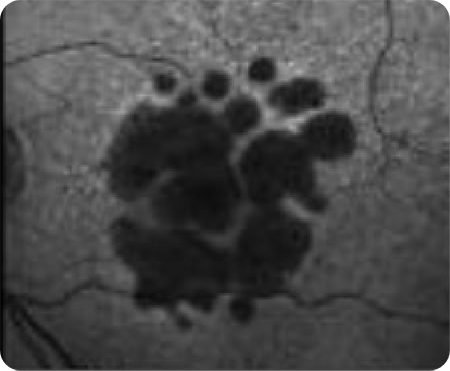
Large -
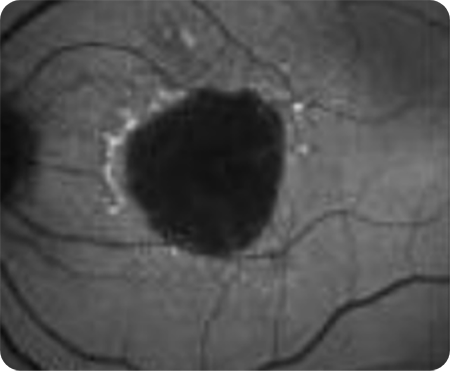
Unifocal 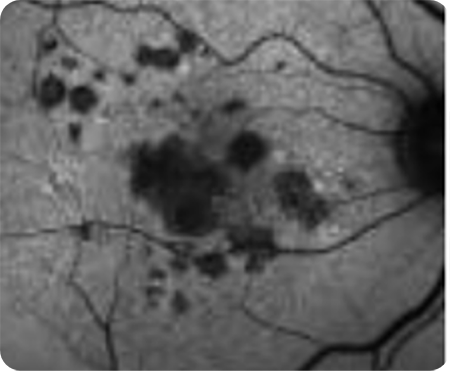
Multifocal -
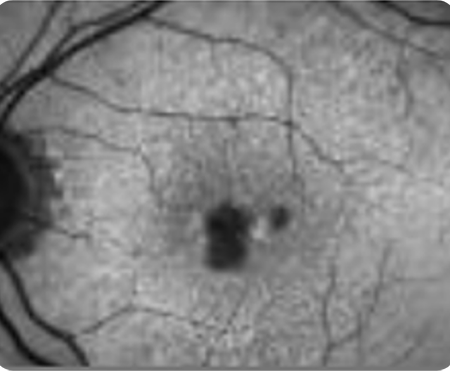
With subfoveal involvement 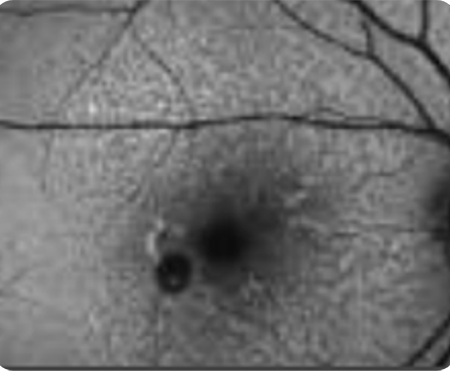
Without subfoveal involvement -

Focal 
Patchy 
Banded 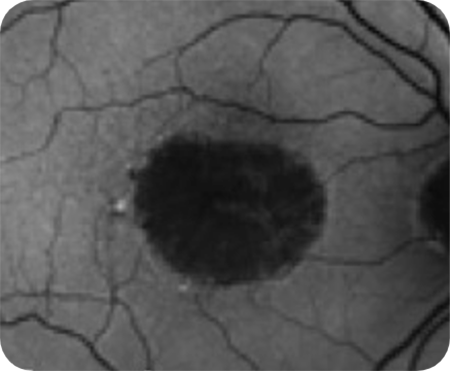
Diffuse 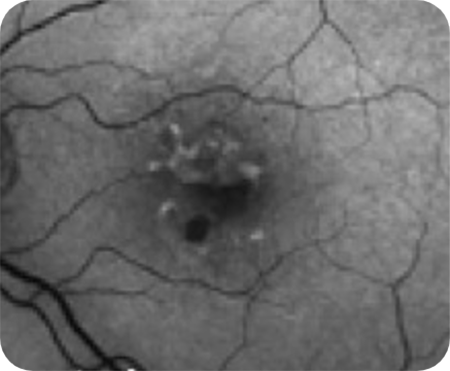
Diffuse-trickling Diffuse and banded patterns are associated with a higher risk of disease progression.
Images may vary based on different device manufacturers and imaging platforms.
Geographic Atrophy (GA): Visual acuity is poorly correlated to lesion size19,20
- Change in visual acuity (VA) may not fully capture disease progression19,20
- Visual function continues to decline as lesions grow18,20,21

80-year-old woman
Hypothetical patient
Medical history:
- No family history of AMD
- BMI 28
- Nonsmoker with exposure to secondhand smoke
- Diabetes, hypertension
- A large area of GA is present at baseline examination. However, BCVA is relatively unaffected due to foveal sparing
- Within 4 years, OS GA has progressed, but BCVA has only declined slightly as fovea is still intact
Baseline
- BCVA: 20/25
- Visual function: Patient requires assistance from a caregiver on some activities (eg, cooking, driving)
NIR and OCT B-scan
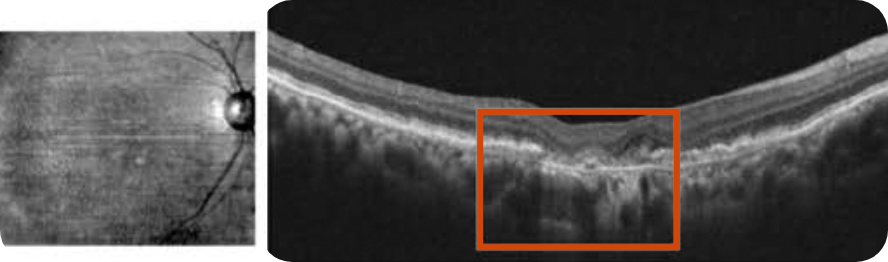
Despite significant atrophy, the fovea is still partially intact
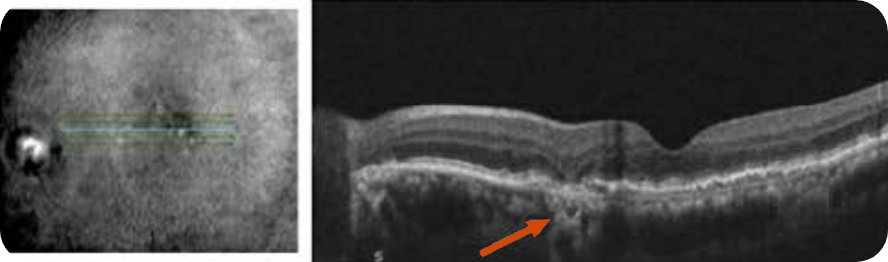
Hypertransmission defect outside the fovea; subsidence of OPL and INL can be seen around the area of atrophy
CFP
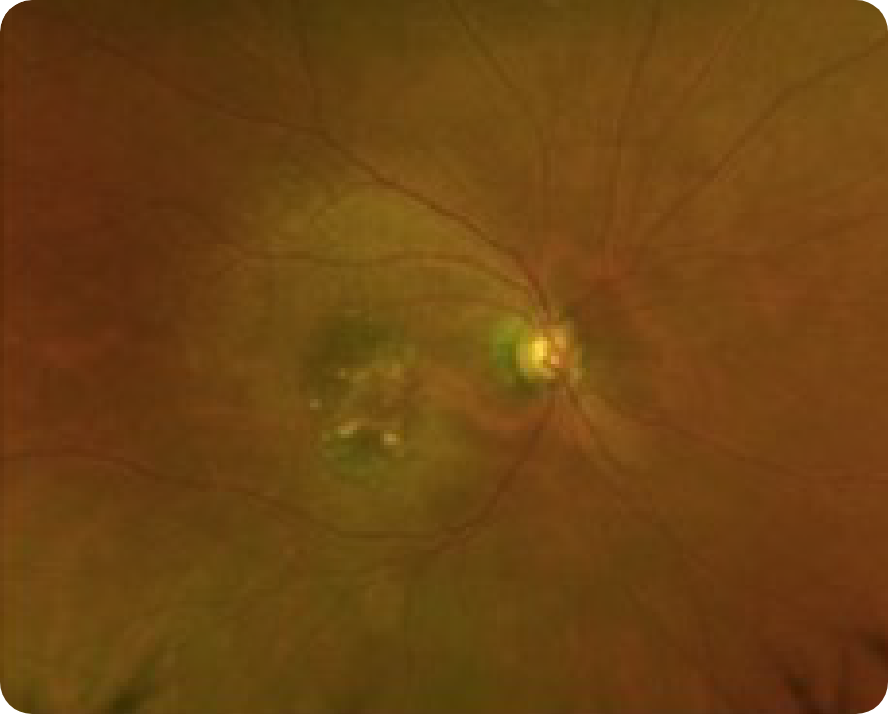
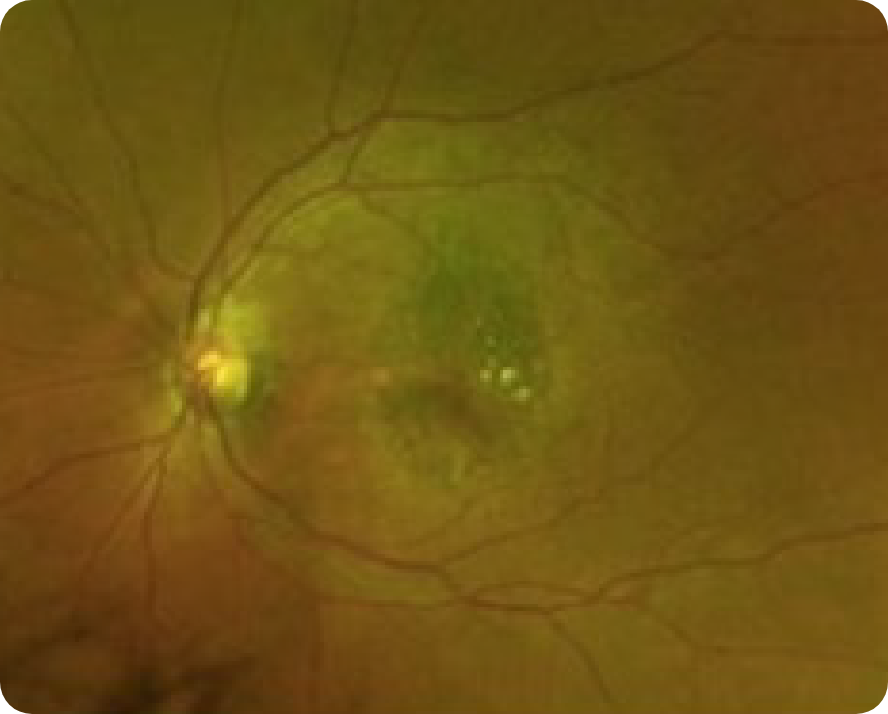
2 Years After (2020)
- OD: 20/100
- OS: 20/25
- Visual function: Reports seeing horizontal lines in inferior field while watching television OD
OCT B-scan and en face
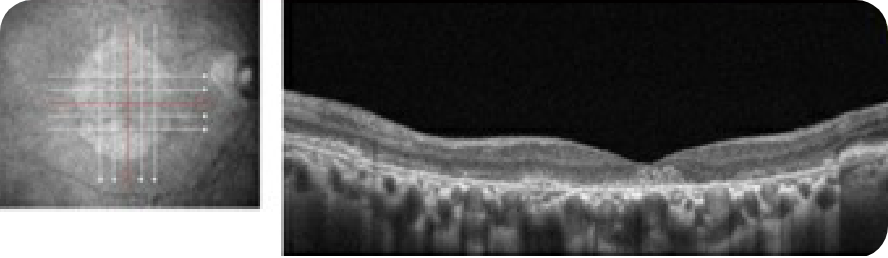
Complete RPE and outer retinal atrophy (cRORA) parafoveally
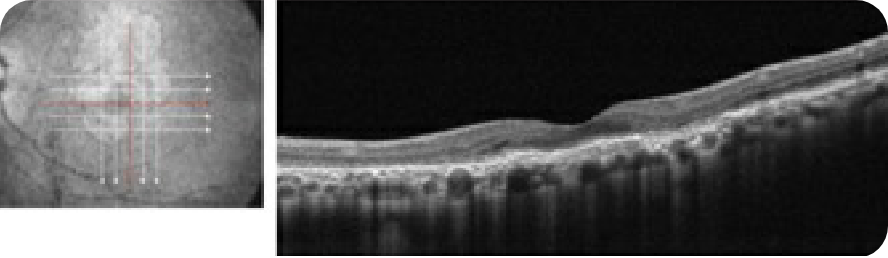
cRORA with choroidal hypertransmission defect. Visual acuity is still somewhat preserved due to partial foveal sparing
CFP
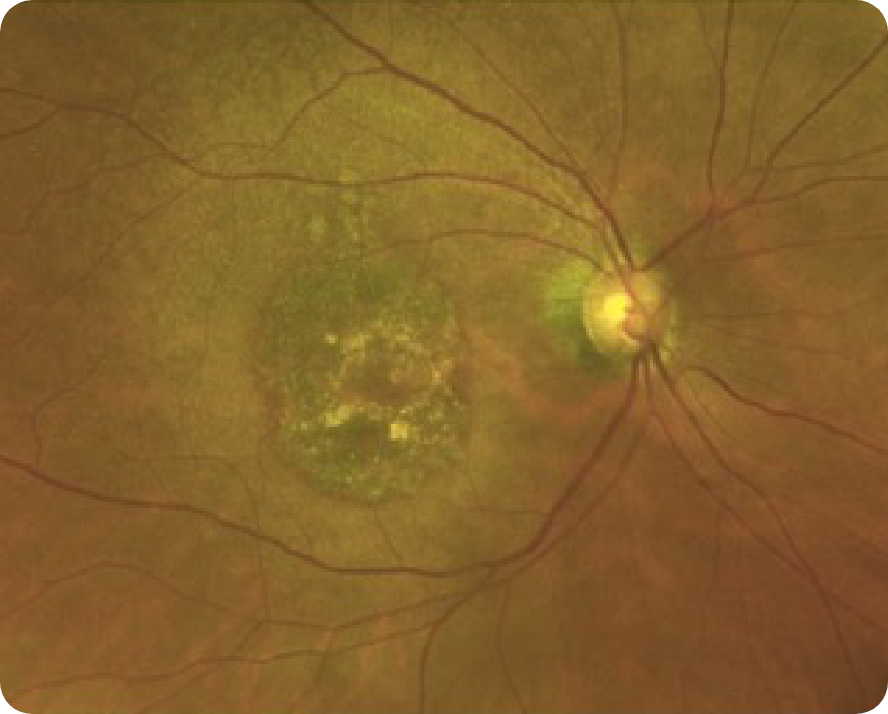
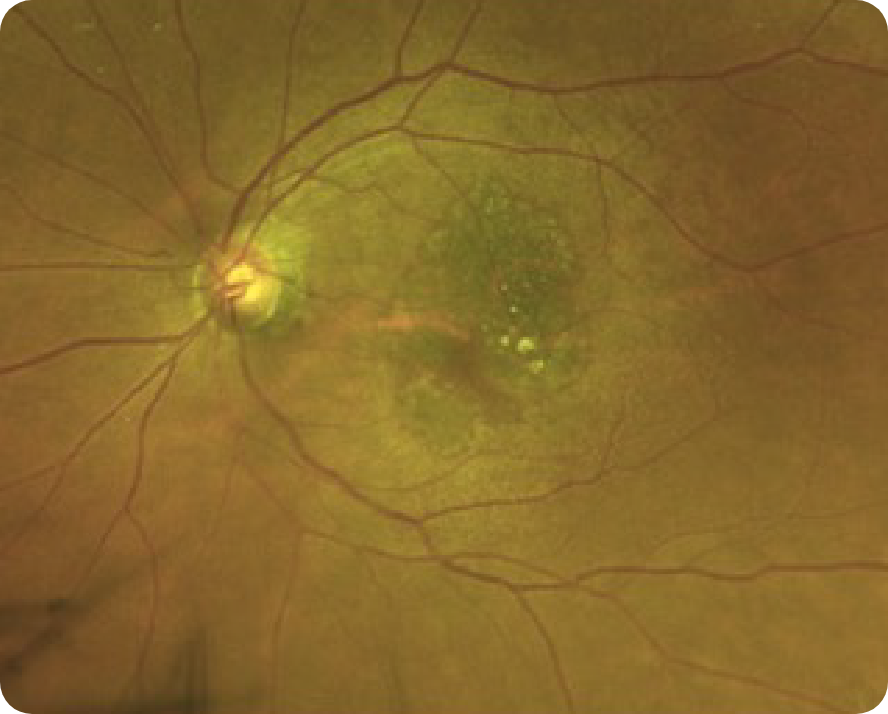
Images may vary based on different device manufacturers and imaging platforms.
OCT and FAF can provide better visualization of Geographic Atrophy (GA) than CFP7,11,12
- Change in visual acuity (VA) may not fully capture disease progression19,20
- Visual function continues to decline as lesions grow18,20,21

71-year-old man
Hypothetical patient
Medical history:
- Current smoker (40 years); smokes 1 pack/day
- BMI 37
- Family history of AMD
- BCVA OD: 20/30
- Patient at baseline has RPE mottling, drusen, and parafoveal patches of atrophy with minimal foveal involvement on CFP OD
- Visual function: Patient reports decreased vision, particularly at night, and “pinhole-like” black spots in his center vision
CFP
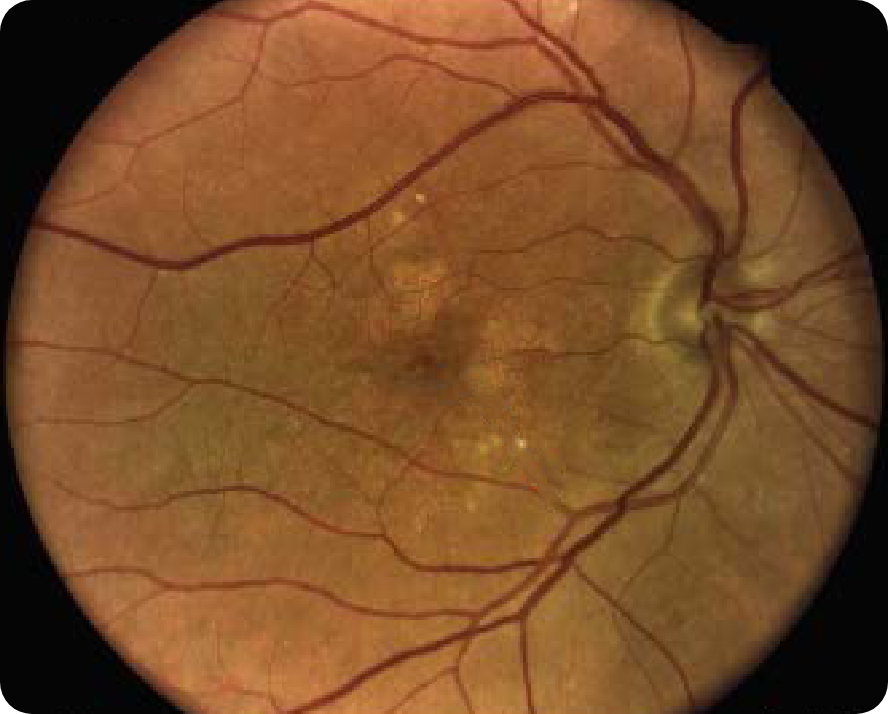
Shows some area of GA
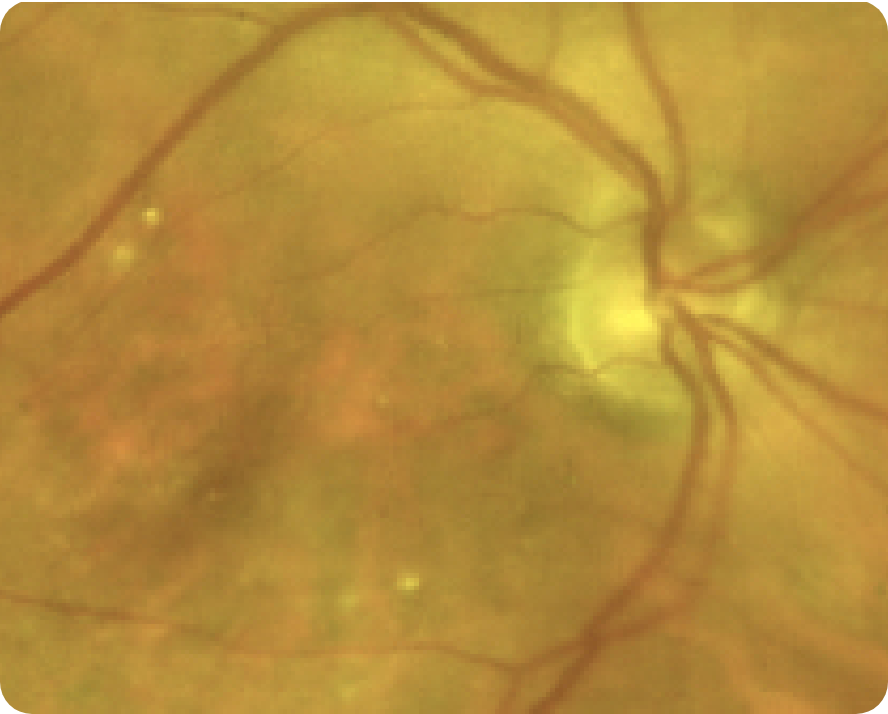
GA is less apparent
OCT B-scan
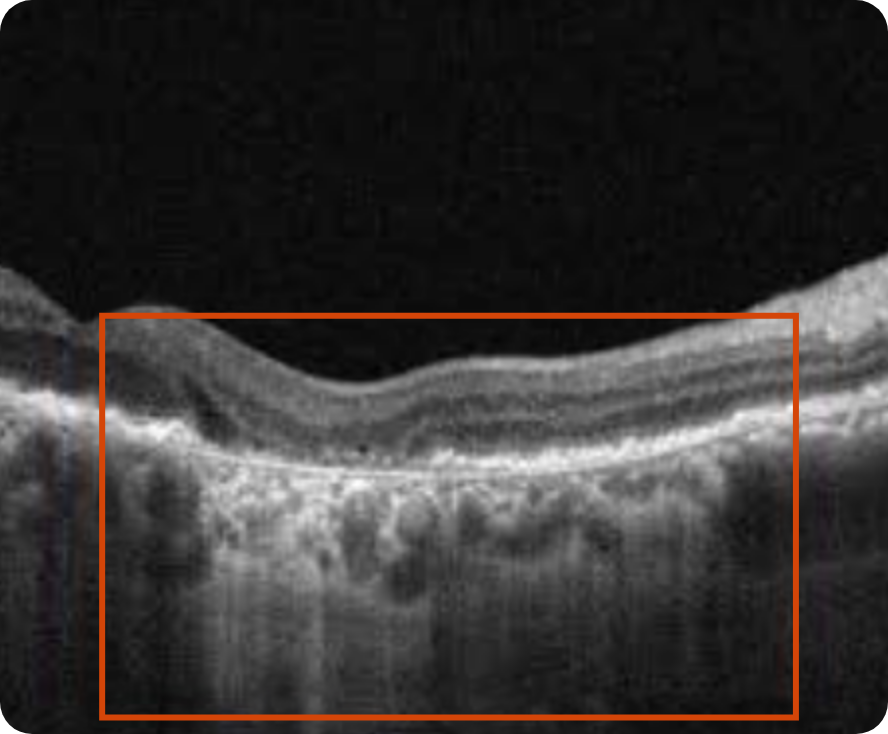

Complete RPE and outer retinal atrophy (cRORA) with increased hypertransmission into choroid
FAF
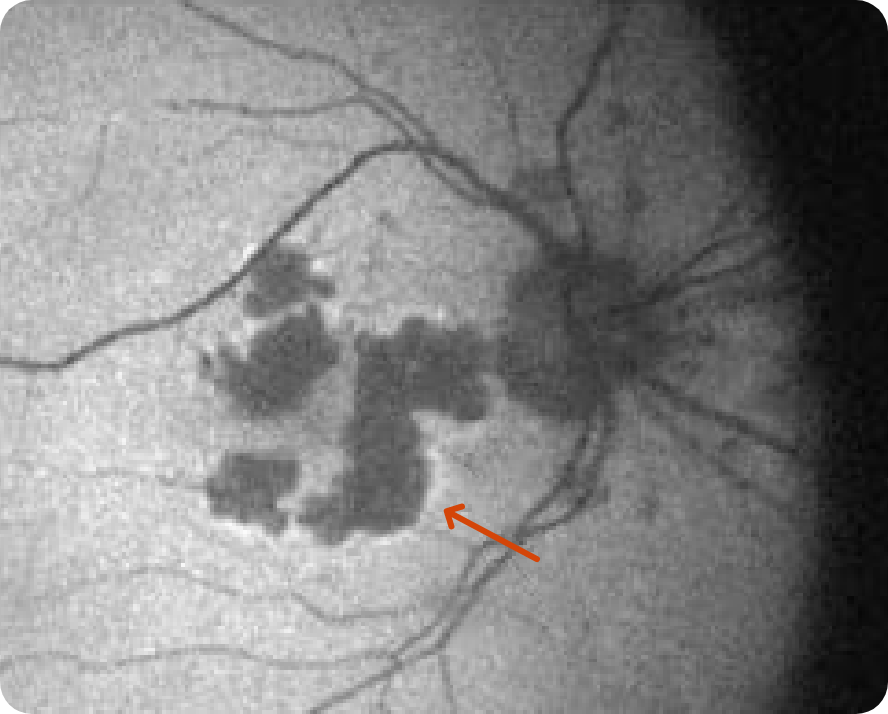
Lesion without subfoveal involvement; hyperfluorescent border around the GA lesions indicating areas that are at risk for further progression
- GA is apparent in both OCT and FAF images
- On OCT, there is the presence of hypertransmission and an area of complete atrophy
- CFP does not show the full extent of GA, and the severity of the disease can be underestimated when only using CFP
Images may vary based on different device manufacturers and imaging platforms.
Geographic Atrophy (GA): Visual acuity is poorly correlated to lesion size19,20
- Change in visual acuity (VA) may not fully capture disease progression19,20
- Visual function continues to decline as lesions grow18,20,21

80-year-old woman
Hypothetical patient
Medical history:
- No family history of AMD
- BMI 28
- Nonsmoker with exposure to secondhand smoke
- Diabetes, hypertension
- A large area of GA is present at baseline examination. However, BCVA is relatively unaffected due to foveal sparing
- Within 4 years, OS GA has progressed, but BCVA has only declined slightly as fovea is still intact
Baseline
- BCVA: 20/25
- Visual function: Patient requires assistance from a caregiver on some activities (eg, cooking, driving)
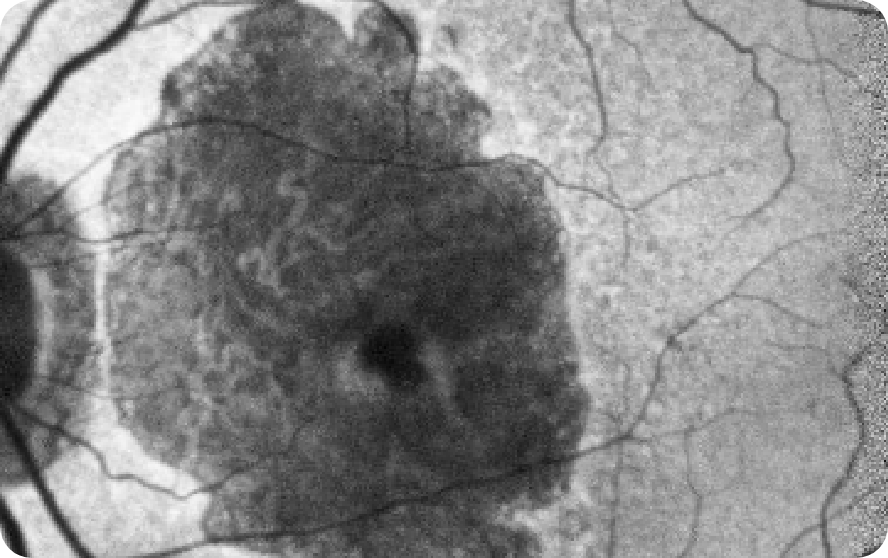
FAF
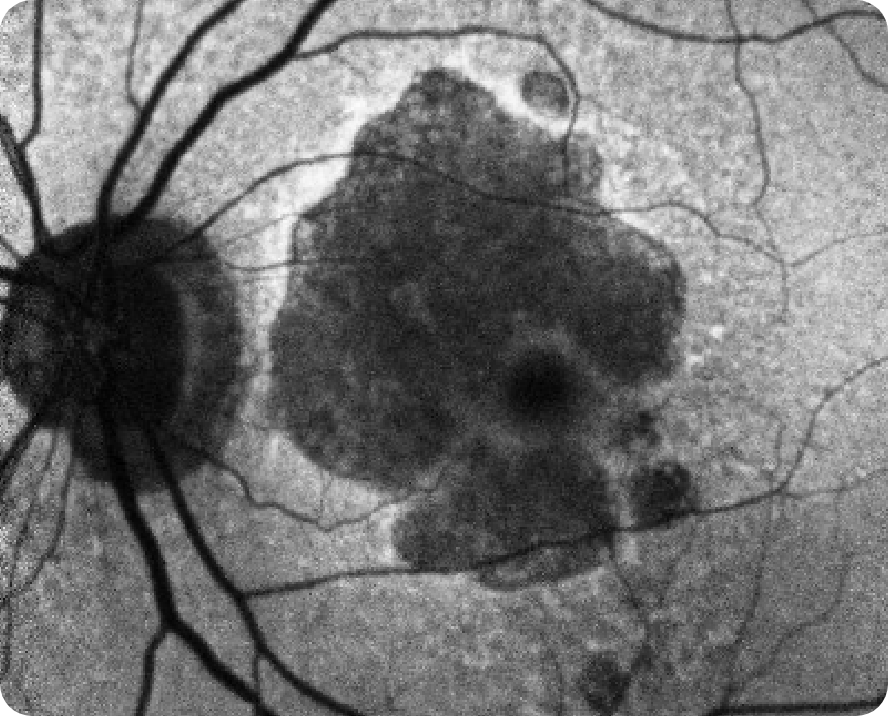
Despite significant atrophy, the fovea is still partially intact
NIR
CFP
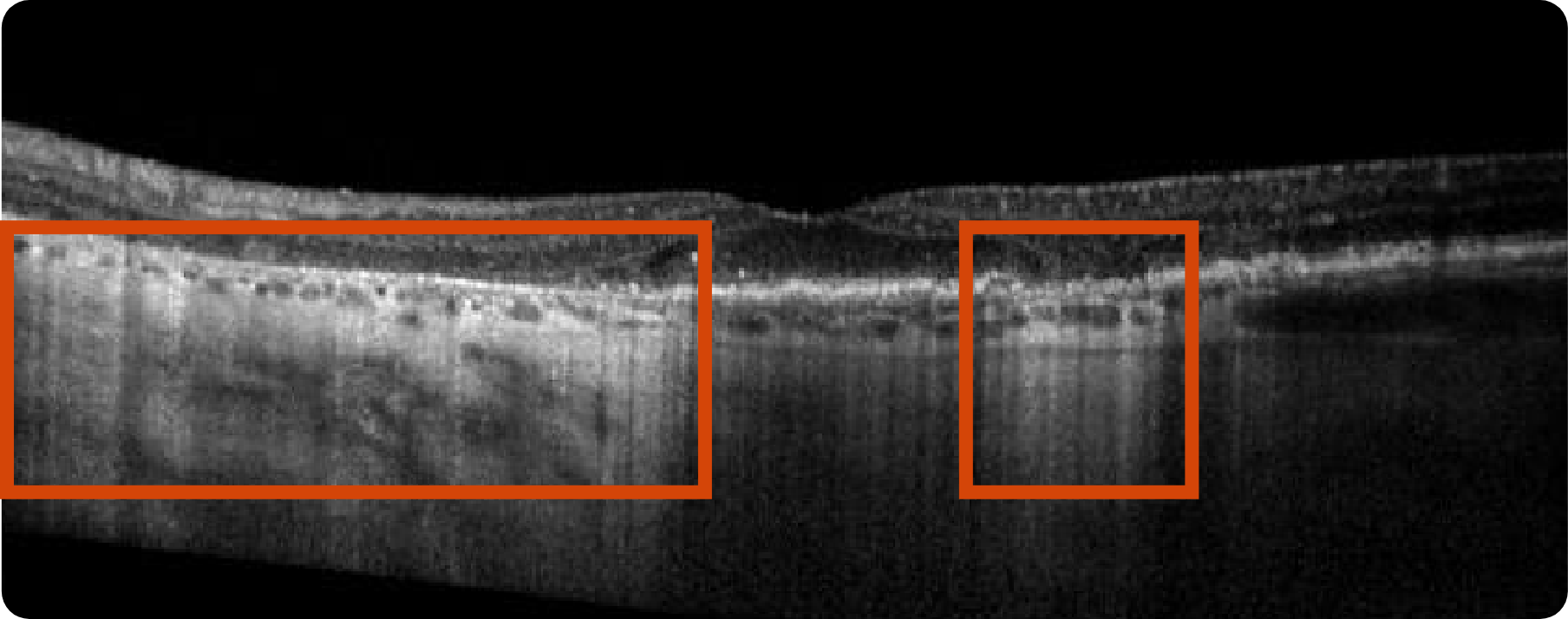
Significant atrophy outside the fovea as shown by choroidal hypertransmission defect
4 Years After
- BCVA: 20/150
- Visual function: Patient has stopped driving, and has trouble reading and seeing faces
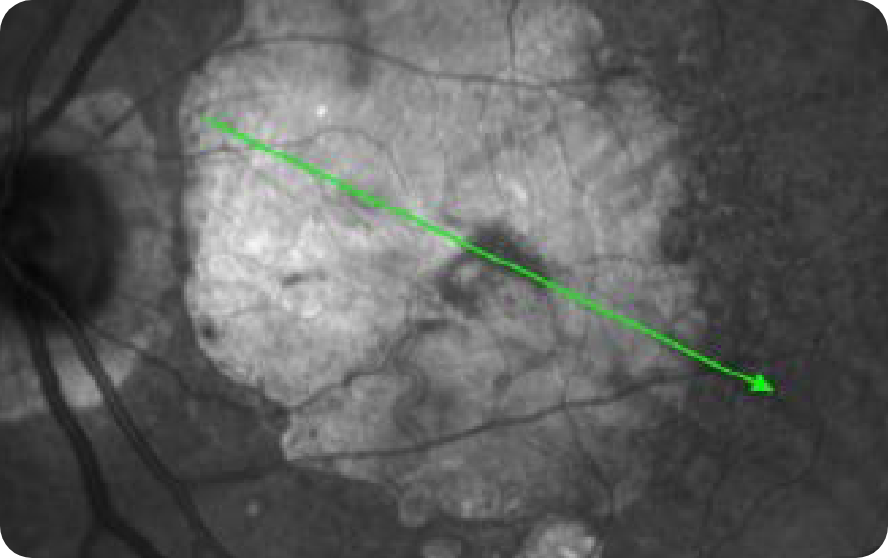
FAF OS
NIR OS
OCT OS
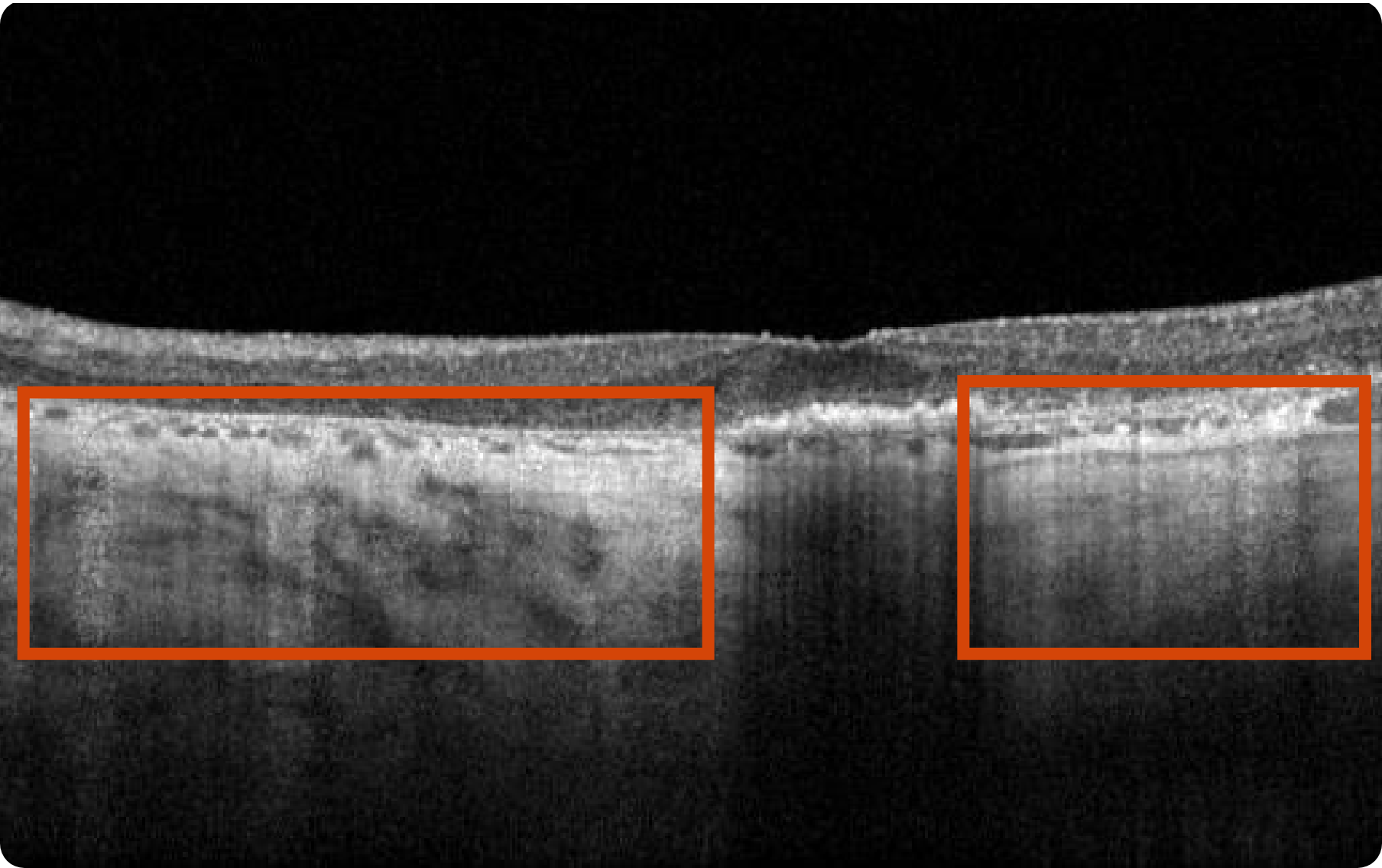

Area of atrophy has grown, as shown by larger region of choroidal hypertransmission. The fovea still remains relatively spared
Images courtesy of Mohammad Rafieetary, OD, Charles Retina Institute.
See how GA may impact your patients
OCT header image courtesy of Dr Julie Rodman, OD, Broward Eye Care Institute.
AMD=age-related macular degeneration; BCVA=best-corrected visual acuity; OCT=optical coherence tomography; RPE=retinal pigment epithelium.
*Baseline and Year 4 images courtesy of Dr Mohammad Rafieetary, OD, Charles Retina Institute.
†The Age-related Eye Disease Study (AREDS) #26, a long-term, multicenter, prospective study examining the progression of GA area in a cohort of 3640 patients with signs of early and more advanced forms of AMD.3
‡The global Geographic Atrophy Insights Survey (GAINS) was sponsored by Apellis and conducted by The Harris Poll between October 12 to December 10, 2021. To accommodate visually impaired respondents, the survey was conducted online and via the telephone among 203 participants aged 60 or over (mean age 70 years) residing in the US, UK, France, Germany, Italy, Netherlands, Sweden, Canada, and Australia who self-reported that they have been diagnosed with age-related macular degeneration (AMD) and have dry AMD in at least one of their eyes. They must also have indicated that they have advanced atrophic age-related macular degeneration or advanced atrophic AMD, advanced/late/late-stage dry age-related macular degeneration or advanced dry AMD, or geographic atrophy (GA) in one or both of their eyes. Included patients must have been currently experiencing at least 3 GA symptoms and currently do/used to do/or have been suggested by an eye care professional but have not done at least one of the following: Take a high-dose formulation of antioxidant vitamins and minerals, stop smoking, maintain a healthy weight and exercise regularly, choose a healthy diet, manage other medical conditions, have check-ups of the retina regularly, or wear sunglasses with UV protection. Included patients must not have been diagnosed with glaucoma, Stargardt disease, or dementia, or be receiving regular injections into the affected eye every 4 to 6 weeks.11