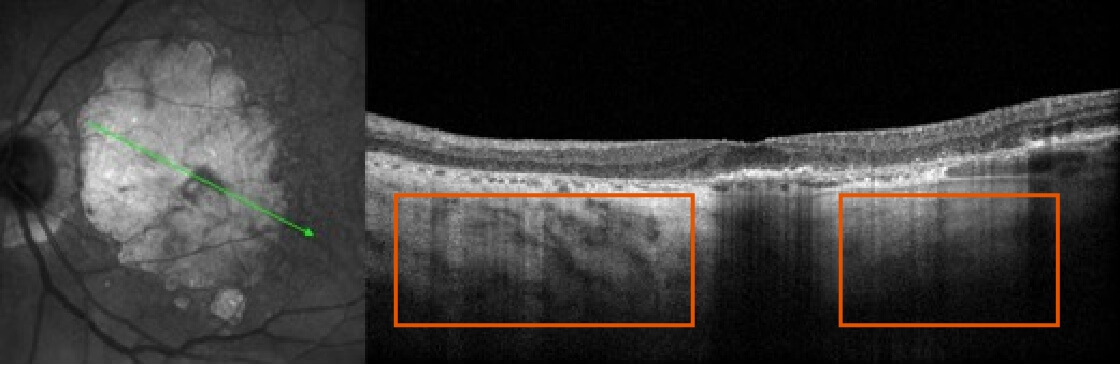
SEE THE SIGNS OF GEOGRAPHIC ATROPHY (GA)
GA can destroy so much





It is critical to recognize GA and refer patients in a timely manner, as disease progression is relentless and irreversible1,4-8
Learn how to recognize GA
OCT=optical coherence tomography.